Portable electronic products with battery as a power source. With the rapid development of portable products, all kinds of the dosage of the battery, and developed many new type of battery. In addition to all the familiar of high-performance alkaline batteries, rechargeable nickel cadmium battery, nickel metal hydride batteries, and in recent years, the development of lithium battery. Here introduces about the basic knowledge of lithium-ion batteries. Including its features, main parameters, the type, application scope and the significance of using matters needing attention, etc.
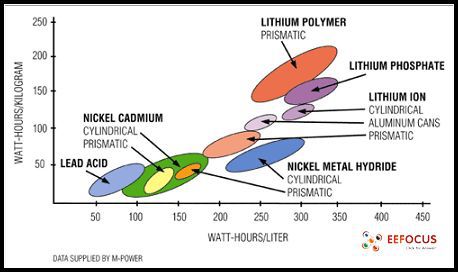
The energy density of a variety of cell types
Lithium is a kind of metal elements, chemical symbol for Li (the English called lithium), is a silvery white, very soft and lively metal chemical performance, is the lightest in the metal. It besides used in atomic energy industry, can manufacture special alloy, special glass (TV screen glass) and lithium batteries. It is used as the battery in the lithium battery anode.
Lithium-ion batteries are divided into two categories: not charging and rechargeable ones. Non-rechargeable batteries as disposable batteries, it can only be chemical energy into electrical energy at once, can't electricity revert to chemical energy (or reduction performance is poor). The rechargeable battery is called secondary battery (also known as the battery). It can transform electric energy into chemical energy storage, when using, and then convert chemical energy into electrical energy, it is reversible, such as the main characteristics of electricity chemical energy lithium batteries.
乐发9 Lithium ion battery cathode material usually has the activity of lithium compounds, the cathode is the special molecular structure of carbon. Common cathode material of main ingredients for LiCoO2, charging, and the positive of the poles of the battery electric potential force compound release lithium ion, embedded negative molecules are arranged in the layer structure of carbon. Discharging, lithium ions from the layer structure of carbon precipitation, and the anode compounds combining again. Lithium ion current of mobile.
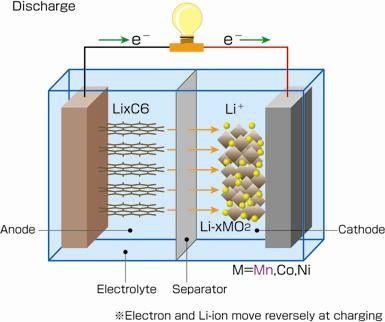
乐发9 Although the chemical reaction principle is very simple, but in the actual industrial production, more need to consider the practical problems: the anode material need additives to be many times the activity of inflation, the cathode material structure at the molecular level to design to accommodate more lithium ions; Filling between is the cathode electrolyte, in addition to the stable, also need to have good electrical conductivity, reduce the battery internal resistance.
乐发9 Although lithium ion batteries have few nickel cadmium battery memory effect, the principle of memory effect is crystallization, almost won't produce this kind of reaction in the lithium battery. However, lithium ion batteries in the many falls and put the filling capacity will still be the reason for this is complex and diverse. Mainly is the change of the anode materials itself, from the molecular level, is the cathode for lithium ion hole structure will gradually collapse, congestion; Anode materials from the chemical point of view, is the activity of the passivation, appear side effects to generate stable other compounds. The physical will also appear on the anode material gradually spalling, etc., in short eventually reduce the battery is free to move in the process of charging and discharging lithium ion number.

